For computerized system we mean a documented verification and high degree of assurance that a specific computerized system performs according to its specifications and quality attributes. Or, A system does what it is expected to do, and this performance can be documented.
DHA Technologies delivers leading Compliance and Computer System Validation services that are designed to help reduce the overall cost of compliance for Pharmaceutical and Life Sciences organizations. We offer our clients cost-effective and comprehensive compliance and validation services.
If you are looking for strategic and sustainable changes in your approach to Computer System Validation and Compliance, our CSV testing team can provide your solution in the area of Computer System Validation and process improvement which will allow organizations to address compliance and CSV issues and reduce costs. We offer validation solutions depending on your specific need, we can support your project with any or all of the services.
Computer System Validation (CSV)
We provide validation services for automated manufacturing practices used in pharmaceutical industries. Having undertaken several validation projects makes us proficient service provider to take care of regulatory requirements.
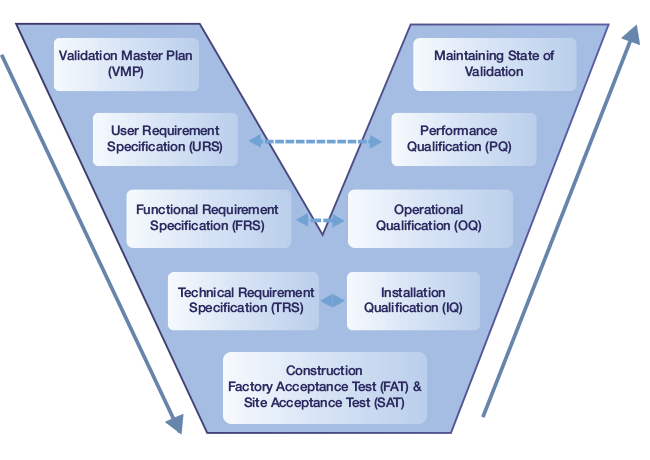
We have good expertise in validation documentation and validation execution. We use a Risk based approach of GAMP 5 for validation.
DHA Technologies can help Life Sciences companies ensure compliance and reduce costs. Our validation consultant/team provides a services for GMP, GCP and GLP application used in Pharmaceutical, Medical Device Industries and Biopharmaceutical Industries. Our background in technology and compliance puts us in an advantageous position to meet our client’s validation needs.
- PLC based control system
- Supervisory Control and Data Acquisition (SCADA) system
- Data Acquisition System (DAS)
- Building Management System (BMS)
- Distributed Control System (CSV)
- HPLC, GS, UV, FTIR, SAS, Chem Station, Winnonlin Software etc.
- Database Software, LCMS software
- Spreadsheet (Excel Sheet)
- ERP / SAP
- MIS / Warehouse Management System
- Validation Project Plans
- Validation Master Plans
- Mitigation Plan
- User Requirement Specification (URS)
- FRS / SRS
- Standard Operating Procedures (SOPs)
- GAP Analysis / GxP Assessment
- Validation Project Plans
- Validation Master Plans
- Mitigation Plan
- User Requirement Specification (URS)
- FRS / SRS
- Standard Operating Procedures (SOPs)
- GAP Analysis / GxP Assessment
- Compliance with regulatory requirements /Regulatory mandates (FDA)
- Ensure the accuracy, reliability, quality and integrity of data
- Minimized risk of malfunction
- Reduction in cost of continuousoperation
- Increased knowledge of processes through improved system knowledge
- Greater trust in computerized systems
